Bridging the Gap: How to Access Special Medical Support When Options Run Out
Navigating the Safety Net: Who Qualifies for Special Medical Support?

The journey for patients living with severe, chronic, or rare diseases often involves navigating a complex web of medical, financial, and emotional challenges. While standard healthcare systems provide essential care, special support is often required for those with the highest unmet needs.
This specialized support is typically available for:
- Patients with Rare Diseases: Conditions that affect a small percentage of the population (Orphan Diseases). Because of their rarity, treatments (known as orphan drugs) are often slow to be developed and approved, creating a critical time gap for patients.
- Individuals with High-Cost, Intractable Conditions: Those whose medical expenses far exceed standard coverage limits, or whose conditions are chronic, debilitating, and have no local approved cure.
- Individuals Ineligible for Clinical Trials: Patients who urgently require a novel therapy but do not meet the strict inclusion/exclusion criteria for ongoing clinical research.
For these patient groups, the standard drug approval and reimbursement processes are often too slow. When all locally approved therapies have been exhausted, the focus shifts to special access pathways, with the Named Patient Program (NPP) being a critical option.
A Lifeline of Last Resort: Understanding the Named Patient Program (NPP)
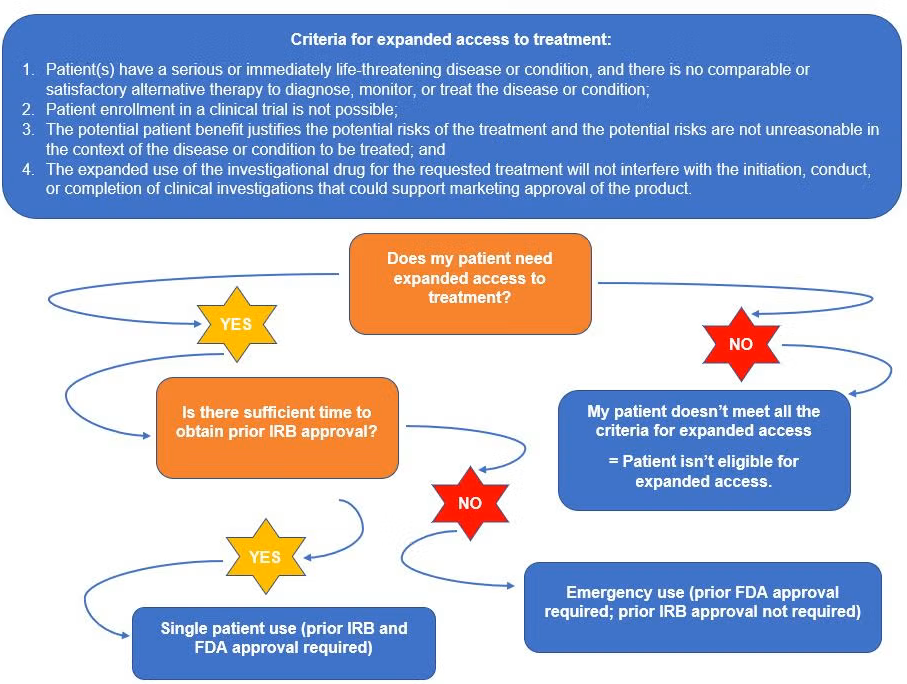
The Named Patient Program (NPP), often known globally as Expanded Access or Compassionate Use, is a strictly regulated pathway that provides hope for patients facing life-threatening or seriously debilitating conditions.
What is the NPP?
The NPP is a legal mechanism that allows a treating physician to request a specific, unauthorized or unapproved medicine for a single, identified patient (hence, "Named Patient").
The drug must meet specific criteria: it is usually undergoing late-stage clinical trials or is already approved and commercially available in at least one other country, but not in the patient's home country.
🔑 Key Eligibility Criteria (for Physicians to Consider):
- Life-Threatening/Severe Illness: The patient must have a condition that is critical or severely debilitating.
- Lack of Alternatives: All locally available and approved therapeutic options must have been exhausted or deemed medically unsuitable.
- No Clinical Trial Enrollment: The patient must be unable to participate in a current, relevant clinical trial.
- Regulatory Oversight: The application must be submitted to and approved by the national health or regulatory authority (e.g., the FDA, EMA, or local equivalent) on a case-by-case basis.
⚠️ A Note on Compliance and Safety
It is crucial to understand that NPP drugs are investigational medicines. Access through an NPP involves an informed risk assessment.
- The NPP is not a promotional tool for the drug.
- The physician assumes full responsibility for the patient's use of the unapproved product.
- The patient must provide fully informed consent, understanding that the drug's long-term risks and benefits may not be fully established.
Our Commitment: TRACKCIRCLE and Global Access Solutions

At Trackcircle, our mission is to ensure that geography and regulatory bottlenecks do not stand between patients and necessary treatments. We understand the urgency, complexity, and emotional toll the early access process places on patients and their doctors.
How We Provide Essential Support:
- Regulatory Navigation: We assist healthcare providers in understanding and complying with the diverse and complex national NPP regulations, managing all required documentation.
- Sourcing and Logistics: We coordinate with licensed international suppliers and pharmaceutical companies to ensure the secure, compliant, and timely sourcing and delivery of the unauthorized medicine, often requiring strict cold-chain logistics.
- Bridging the Gap: We act as the ethical and professional intermediary, connecting physicians with the global supply chain, ensuring end-to-end compliance and transparency.
If you are a treating physician seeking access to a critical therapy for a patient with no local alternatives, or a patient's family member seeking information on the NPP process, we are here to help you navigate the process.
Disclaimer
This content is for informational and educational purposes only and does not constitute medical advice, nor is it intended to promote any specific investigational drug. Access to the Named Patient Program (NPP) is strictly managed by the patient’s treating physician and national regulatory bodies. Individual treatment outcomes and risks will vary.

